Vagus Nerve Stimulation (VNS) for Treating Depression
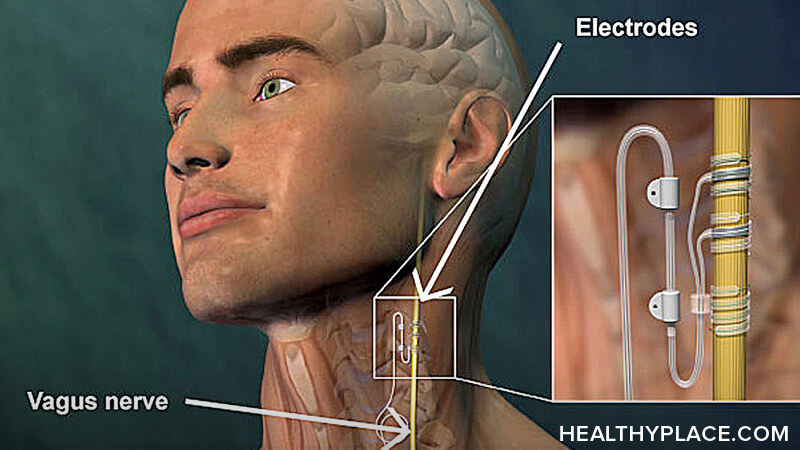
Vagus nerve stimulation is a treatment for depression and seizure disorders that involves the electrical stimulation of the vagus nerve. For vagus nerve stimulation, a generator, wires and electrodes are implanted to deliver electricity to the vagus nerve at predetermined intervals.
In July 2005, vagus nerve stimulation (sometimes called vagal nerve stimulation) was approved by the FDA for the treatment of depression in treatment-resistant patients. To meet the criteria for vagus nerve stimulation therapy (VNS therapy), a patient must:
- Be age 18 or older
- Have treatment-resistant depression
- Have chronic depression that has lasted two or more years
- Have depression that hasn't improved after the use of at least four or more antidepressants or ECT or both
Vagus nerve stimulation (VNS) doesn't guarantee an improvement in depression symptoms. The FDA advises vagus nerve stimulation must be used in addition to traditional depression treatments such as antidepressants.
Does Vagus Nerve Stimulation for Depression Work?
FDA approval was given to VNS therapy based on two studies: one pilot 10-week study with 60 participants and one sham or placebo-controlled 10-week study with 235 participants. In both VNS studies, patients were being treated for severe, refractory depression.1
- In the pilot study approximately 30.5% of patients responded to treatment and 15.3% remitted
- In the larger study, 15.2% of patients responded to treatment while 10% of patients responded to sham (placebo) treatment
- Patients in both studies were followed and at one year after device activation, positive response rates were approximately 43% in both study groups
FDA approval of VNS continues to be controversial with some professionals indicating the study on which approval was based was flawed.
Vagus Nerve Stimulator Implantation
Surgery to implant the VNS device may be done on an inpatient or outpatient basis. Surgery is typically done under a general anesthetic. A VNS electrical pulse generator (battery-powered) is surgically implanted under the skin in the chest and wires connected to it are guided up and wrapped around the left vagus nerve.
Vagus Nerve Stimulation Side Effects
Side effects for vagus nerve stimulation can come from the implantation surgery, the vagus nerve stimulation itself, or both. Vagus nerve stimulator implantation and VNS therapy are both considered safe but complications can occur. VNS surgery side effects include:
- Pain / scarring at the incision sites
- Infection
- Damage to the vagus nerve
- Breathing problems
- Nausea
- Heart problems
- Vocal cord paralysis, which is usually temporary
VNS therapy side effects tend to occur only when the pulse generator is actually stimulating the vagus nerve. VNS therapy side effects include:
- Voice changes (in over half the people who have the procedure)
- Hoarseness; cough
- Throat or neck pain
- Chest pain or spasms
- Breathing problems, especially during exercise
- Difficulty swallowing
- Tingling or prickling of the skin
VNS Implant Costs
One of the major downsides of vagus nerve stimulation therapy is the cost of implantation and treatment. Not only are there costs to having the VNS device implanted but there are additional costs as the device must be monitored and programmed regularly by a doctor.
The cost of implanting a VNS device is approximately $30,000 and up. Medicaid does not pay for VNS2 although some healthcare insurance companies will pay for it on a case-by-case basis.3
More details about vagus nerve stimulation, implantation, costs, insurance coverage and obtaining treatment can be found through the Cyberonics web site: http://depression.cyberonics.com/depression/main.asp
APA Reference
Tracy, N.
(2022, January 4). Vagus Nerve Stimulation (VNS) for Treating Depression, HealthyPlace. Retrieved
on 2024, June 20 from https://www.healthyplace.com/depression/depression-treatment/vagus-nerve-stimulation-vns-for-treating-depression



